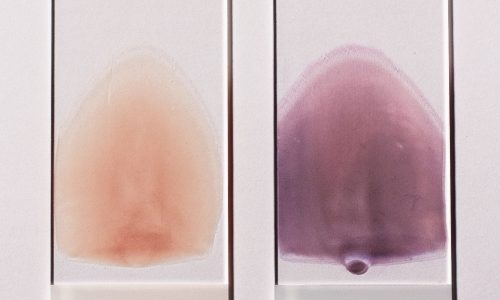
The Wright-Giemsa stain is a Romanowsky stain. Romanowsky stains are said to achieve the Romanowsky effect which is the ability to produce a wide range of hues along a gradient; sometimes also called metachromasia. The Wright-Giemsa should not be confused with other Romanowsky stains such as the Giemsa stain, which is the standard for Malarial testing, nor the Wright’s stain.
Near the 1870s a Methylene blue stain with acid fuchsin was used to stain blood smears, by the 1890s the acid fuchsin was replaced with eosin (this was essentially a Wright’s Stain but wouldn’t be described so for at least another 10 years), and a few years later it was noted by Romanowsky that if the methylene blue dye was aged until ‘mold grew on top’ that the stain would produce a polychrome effect known as none other than the Romanowsky effect. The Giemsa stain supposedly leaves out the Methylene blue but uses is oxidized products known as Azure A, B, C, thionin, and Azure II. It produces a lighter blue-ish cyan color, and doesn’t adequately stain the nuclear elements of a blood smear. With the Methylene blue included the stain is known as Wright-Giemsa, and when the oxidative products are exlcuded (e.g. methylene blue and eosin only) it is known as a Wright’s stain although heat is used to polychrome the methylene blue.
The stain can be produced by dipping slides into coplin jars, using a sliding stainer, or aerosol spray staining. Coplin jars which are the most common manual method the primary stain is the red eosin followed by methanol fixation, and then counterstained with the deep purple wright-giemsa stain. Note that the Eosin is retained in acidic structures and that the Methylen blue is better retained in basic structures. For an additional history and example procedure see the two links below.
Wright-and-Wright-Giemsa-Hematology-Stain
http://www.cpsa.ca/wp-content/uploads/2016/08/Blood-Film-Staining-Effects-Educational-Document.pdf