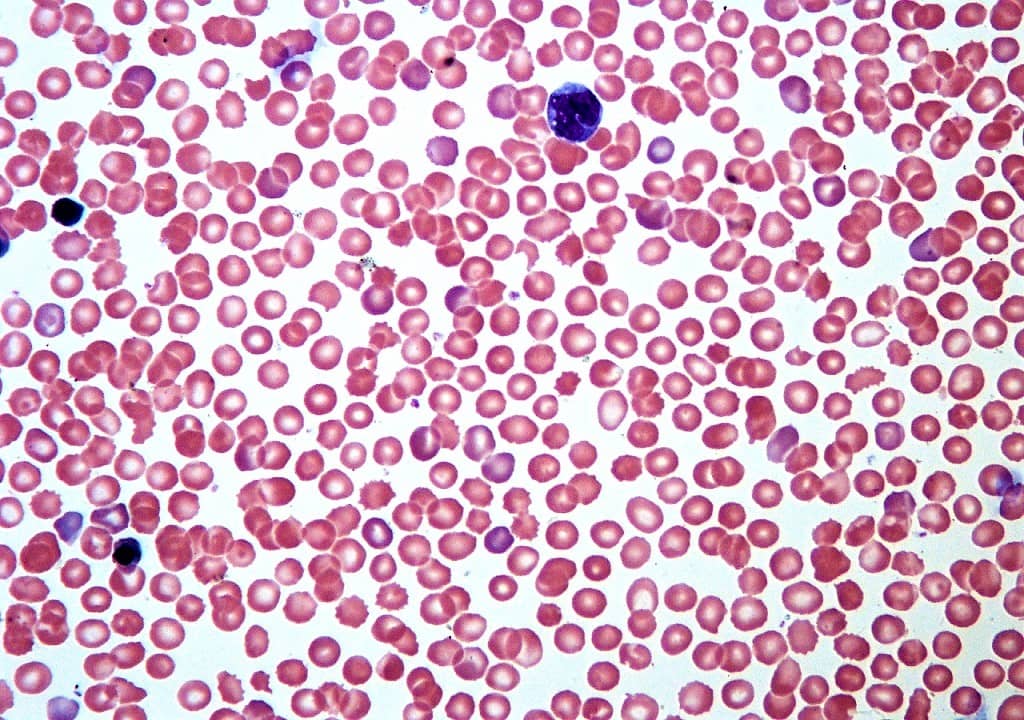
Hyperchromia
Cytochemical Staining of Leukemias
Stain |Site of Action |Cells Stained |Comments |Image
Myeloperoxidase
Primary Granules;
Auer Rods
Myeloblasts; Granulocytes;
Monocytes slight positive
Separates AML+ from AML-
Sudan Black B Phospholipids
Myeloblasts; Granulocytes;
Monocytes slight positive
Separates AML+ from AML-
Naphthol AS-DChloroacetate/(CAE) – specific esterase
Cytoplasm
Neutrophilic granulocytes;
Mast cells
Separates AML+ from AML-
Periodic acid-Schiff Stain (PAS)
Glycogen Granular pattern with negative background in lymphoblasts.
Positive in erythroleukemia, abnormal erythrocyte precursors, and ALL
Also, histochemical staining for terminal deoxynucleotidyl transferase (TdT) can be useful in determining that the blast is of lymphoid lineage. TdT is an enzyme found in immature (developing) lymphocytes which functions to help synthesize their specific antigen receptors.
Chronic Myeloid Leukemia
BCR-ABL test for Philadelphia chromosome: A gene formed when pieces of chromosomes 9 and 22 break off and trade places.
t (8;21) – Acute Myeloid Leukemia (FAB M2)t (8;14) – Burkitt’s Lymphomat (11;14) – Chronic Lymphoid Leukemia and multiple myeloma
Kohler Illumination and Microscope Calibration
Accute ProMyelocytic Leukemia (APML)
Start ATRA STAT
B cell Lymphoma
AKA Hairy Cell lymphoma
CBC and Auto-Differential
Hemacytometers were the first tools used to perform manual cell counts. A standard ‘charge’ would fill a 3×3 gridded area which was eactly 0.9uL. The coulter counter was one of the first automated laboratory instruments and would count using a tube so skinny exactly one cell could fit through its diameter. A constant electrical charge would be flowing across a part of the cross section and the charge would be impeded by a cell traveling through through. The resistance was measured and directly correlated to cellular volume.
MCHC = HGB * 100 / HCT
MCH = HGB *10 / RBC
MCV = HCT * 10 / RBC
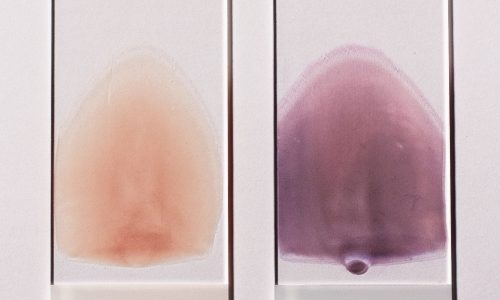
Wright Giemsa Stain
The Wright-Giemsa stain is a Romanowsky stain. Romanowsky stains are said to achieve the Romanowsky effect which is the ability to produce a wide range of hues along a gradient; sometimes also called metachromasia. The Wright-Giemsa should not be confused with other Romanowsky stains such as the Giemsa stain, which is the standard for Malarial testing, nor the Wright’s stain.
Near the 1870s a Methylene blue stain with acid fuchsin was used to stain blood smears, by the 1890s the acid fuchsin was replaced with eosin (this was essentially a Wright’s Stain but wouldn’t be described so for at least another 10 years), and a few years later it was noted by Romanowsky that if the methylene blue dye was aged until ‘mold grew on top’ that the stain would produce a polychrome effect known as none other than the Romanowsky effect. The Giemsa stain supposedly leaves out the Methylene blue but uses is oxidized products known as Azure A, B, C, thionin, and Azure II. It produces a lighter blue-ish cyan color, and doesn’t adequately stain the nuclear elements of a blood smear. With the Methylene blue included the stain is known as Wright-Giemsa, and when the oxidative products are exlcuded (e.g. methylene blue and eosin only) it is known as a Wright’s stain although heat is used to polychrome the methylene blue.
The stain can be produced by dipping slides into coplin jars, using a sliding stainer, or aerosol spray staining. Coplin jars which are the most common manual method the primary stain is the red eosin followed by methanol fixation, and then counterstained with the deep purple wright-giemsa stain. Note that the Eosin is retained in acidic structures and that the Methylen blue is better retained in basic structures. For an additional history and example procedure see the two links below.
Wright-and-Wright-Giemsa-Hematology-Stain
http://www.cpsa.ca/wp-content/uploads/2016/08/Blood-Film-Staining-Effects-Educational-Document.pdf
ESR – Westergren method
Check out this interesting history on ESR: Grzybowski_Ashort_IJLH2012
In general the greater amount of protein in the blood the faster it settles and therefore has a greater ESR. Usually results greater than 60 will be confirmed with a 60 minute ESR, but lower results can be safely reported in as little as 20 minutes and multiplied by a time factor.
High ESR can be caused by:
- Inflammation – for which this test is commonly used as a screening tool
- Infection
- Rheumatoid arthritis
- Rheumatic fever
- Vascular disease
- Inflammatory bowel disease
- Heart disease
- Kidney disease
- Certain cancers (multiple myeloma)
- Females
- Pregnancy
- Old age
- hyperfibrinigenemia
- macrocytosis
Low ESR can be caused by:
- Polycythemia
- Sickle cell anemia
- Leukocytosis
- microcytosis
- hypofibrinogenemia
- hypogamaglobinemia
There are two prevailing methods. The Wintrobe method uses 100mm thin tube whereas the Westergrenn uses a 200mm thicker tube. The modified-Westergrenn uses the same thicker tube but is usually automated and abbreviated to less than 60 minutes.
Lavender top tubes are most commonly used for this methodology and when loaded the automated instrument or method will diluted the sample with sodium citrate in a 1:4 ratio (5X factor). Black top tubes are also acceptable if validated with the testing system. They are sometimes made of glass so need to be handled with extra care. They contain a pre-aliquoted amount of sodium citrate so they must be filled to the correct level and not diluted a second time by the analyzer or protocol.
Sources: https://medlineplus.gov/lab-tests/erythrocyte-sedimentation-rate-esr/ https://www.aafp.org/afp/1999/1001/p1443.html
Photo: https://www.fishersci.com/shop/products/polymedco-sediplast-autozero-esr-system-7/p-190433